Natural Samples of Copper Contain Two Isotopes
These isotopes decay within the rocks according to their half-life rates and by selecting the appropriate minerals those that contain potassium for instance and measuring the relative amounts of parent and daughter isotopes in them the date at which the rock crystallized can be determined. Astatine is a chemical element with the symbol At and atomic number 85.
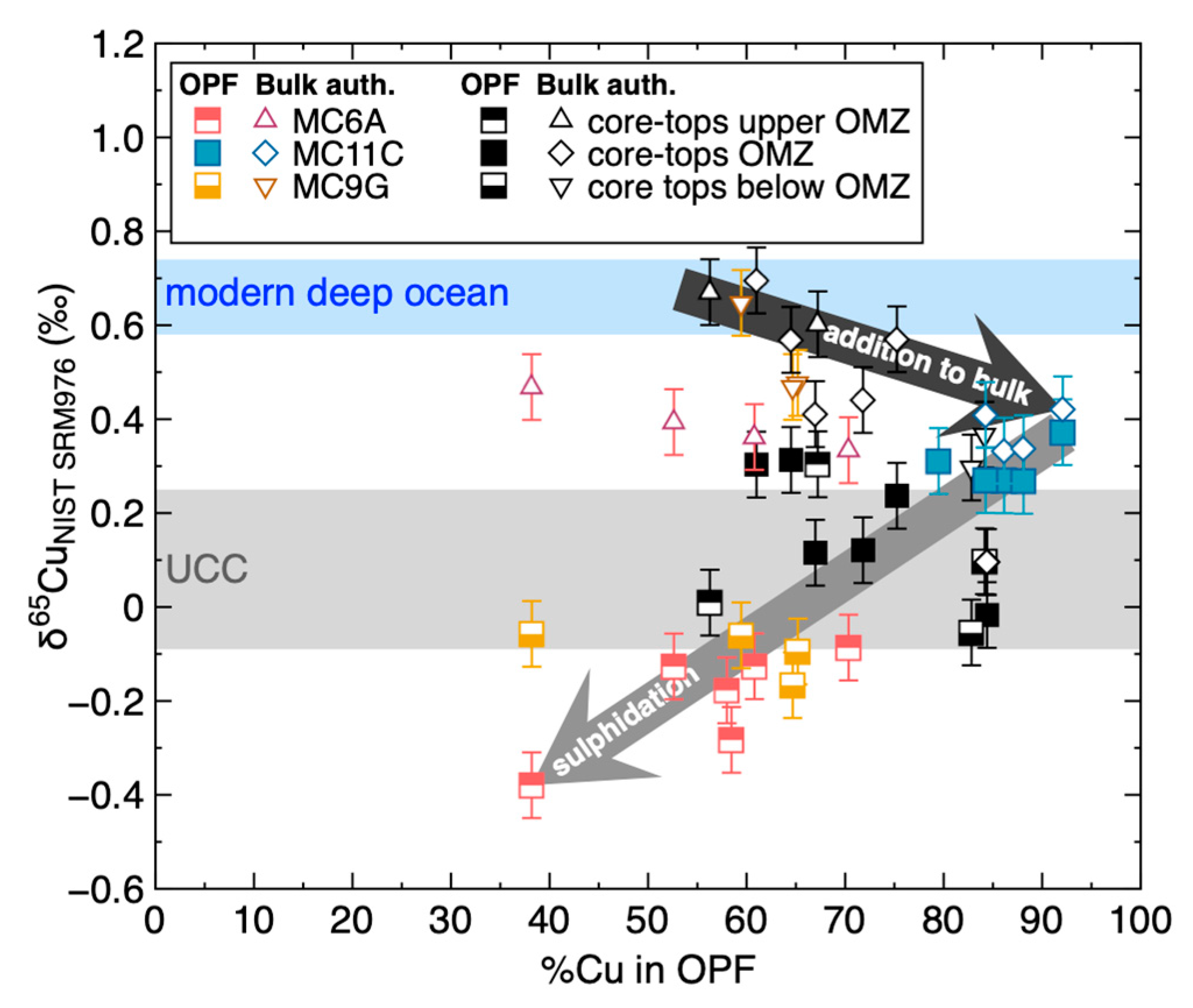
Geosciences Free Full Text Copper And Its Isotopes In Organic Rich Sediments From The Modern Peru Margin To Archean Shales Html
Which isotope has the.

. With atomic number 88 it has four natural isotopes of atomic weight 228 226 224 and 223 - though there are a remarkable 21 more artificial isotopes. Isotope 1 has a mass of 800 amu Isotope 2 has a mass of 850 amu. The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes.
The most stable is astatine-210 with a half-life of 81 hours. Isotopes are atoms with the same atomic number but different mass numbers. ISOTOPES OF HYDROGEN 12.
If the first isotope is 86 amu and the second isotope has a mass of 90 amu. - -- --- ---- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- -----. Copper has only two naturally occurring isotopes Cu - 63 and Cu - 65.
Isotopes of any particular element contain the same number of protons but different numbers of neutrons. A fictional element has two isotopes each making up 50 of the population. The mass of Cu - 63 is 629396 amu and the mass.
ISOTOPES OF CARBON 13. A fictional element has two isotopes and an atomic mass of 8708 amu. A sample of the pure element has never been assembled because any.
All of astatines isotopes are short-lived. A later starring role for radium would be as the source of alpha particles - helium nuclei - used by Rutherford in 1909 at the Cavendish laboratory in Cambridge to fire at a thin gold foil. The second has a mass of 649 amu and a percent natural abundance of 309.
It is the rarest naturally occurring element in the Earths crust occurring only as the decay product of various heavier elements. Calculate the atomic mass of the fictional element. Most of the large igneous rock masses of the world have been dated in this manner.
A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons. A 235 Z 92 Number of protons 92 Number of. U 235 92 U 238 92 There are many isotopes of uranium.
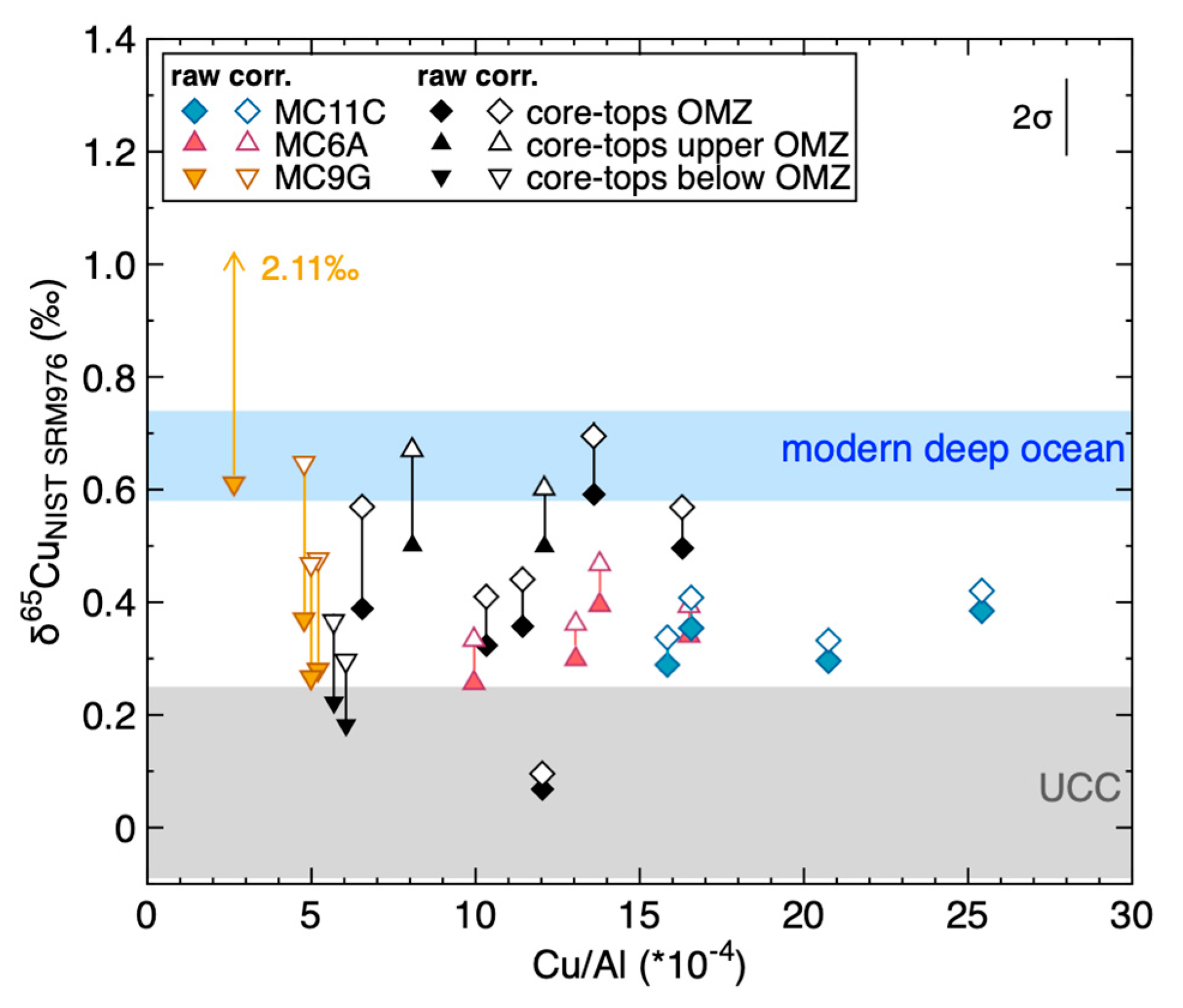
Geosciences Free Full Text Copper And Its Isotopes In Organic Rich Sediments From The Modern Peru Margin To Archean Shales Html
Naturally Occurring Copper Has Two Isotopes With Relative Atomic Masses 62 93 And 64 928 If The Average Relative Atomic Mass Is 63 546 What Are The Relative Abundances Of The Two Isotopes In This Sample Quora

Oxidation Number Example 3 Redox Reactions Oxidation Chemical Reactions
No comments for "Natural Samples of Copper Contain Two Isotopes"
Post a Comment